Accueil > Équipes scientifiques > Structure et dynamique des systèmes complexes isolés photoexcités (SYSIPHE) > CHIralité et spectroscoPIE (CHIPIE) > Vibrational Circular Dichroism
Vibrational Circular Dichroism
par - 31 juillet 2018 (modifié le 25 mars 2020)
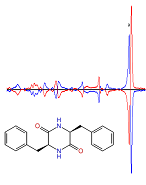
Our recent VCD studies include cyclic dipeptides in the solid phase, like diketopiperazine peptides and those derived of cis or trans 2-aminocyclobutane-1-carboxylic acids (ACBCs).
The cyclo LL diphenylalanine is organised as dimers in the solid state, which are bound by a double hydrogen bridge. This is however not the case for the LD dipeptides for which dispersion between the aromatic rings is the major interaction in the solid phase.

- Solid-state cyclo LL diphenylalanine
Formation of cyclo LD diphenylalanine in the solid phase by thermal peptide bond formation in the linear LD diphenylalanine parent leads to the formation of a new chiral phase resulting from the synchronisation of the transient chirality of the -otherwise not chiral- subunit.
cis or trans ACBC derivatives have been studied in the solid phase. While the VCD spectrum of the cis derivative is described by that of the monomer in the solid phase, that of the trans requires inclusion of at least a tetramer. A dramatic enhancement in the VCD intensity of the trans derivative relative to the cis is observed.

- VCD spectrum and structure of trans and cis ACBC derivatives
We are also inteerested in the description of VCD spectra for molecules in strong interaction with their solvent. The “Cluster-in-the bulk” description of the influence of hydrogen bond formation on VCD spectra allows proposing a computationally affordable strategy for the calculation of VCD spectra of flexible chiral molecules in strong interaction with their environment. It was compared for 1-indanol insolution in DMSO to time-dependent methods resting on first-principles molecular dynamics (MD) simulations that fully account for anharmonicity, temperature and environment effects.





