Accueil > Équipes scientifiques > Structure et dynamique des systèmes complexes isolés photoexcités (SYSIPHE) > CHIralité et spectroscoPIE (CHIPIE) > Chirality and photostability of quinine derivatives
Chirality and photostability of quinine derivatives
par - 4 novembre 2015 (modifié le 25 mars 2020)

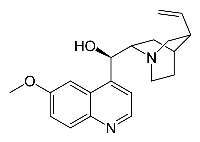
- Quinine
The studied dimers contain a quinine unit as well as one of the pseudoenantiomers, namely, cinchonine or cinchonidine. One can thereby study the homochiral dimer (quinine-cinchonidine) and the heterochiral dimer (quinine-cinchonine)
They have been spectroscopically characterised in the IR range. Their collision-induced dissociation leads to the evaporation of the monomers and shows important chirality effects. The dissociation yield is smaller for the homochiral complex. The photo-fragmentation induced by a UV laser leads to the following products.

- Proposed mechanism of fragmentation after UV photo-excitation
The proposed mechanism have been confirmed by experiments coupling simultanously UV and Free-electron IR or OPO lasers in the ion trap. The UV induces photofragmentation while the IR probes the structure of the products. The two photoproducts have been characterised by their vibrational spectra. In particular, an exoic protonated species with the proton located in the quinoline nitrogen has been shown to be formed by ESIPT (Excited State Proton Transfer)
Combining the structural characterisation of the fragments with quantum chemical calculations, we are able to propose a photofragmentation mechanism which involves two paths :
Excitation ot the protonated moiety of the dimer leads to coupling with a dissociative pi-sigma* state leading to hydrogen transfer combined to electron transfer, acompanied by the cleavage of the adjacent CC cleavage.
Excitation of the neutral moiety leads to Excited StateProton Transfer (ESPT) followed by dissociation of the intermolecular bond.
|
|
In parallel, we have studied jet-cooled neutral quinine by means of IR-UV double resonance spectroscopy. The molecules are put intact in the gas phase thanks to a laser ablation source (picture in Techniques et Développements expérimentaux)

- Most stable structure of the CdH2SO4H+ complex
Complexation with sulfuric acid completely modifies the photophysics of protonated cinchona alkaloids. IRMPD spectroscopy unambiguously demonstrates that the protonated complexes consist of a doubly protonated cinchona alkaloid strongly bound to a bisulfate HSO4- anion, which bridges the two protonated sites of the cinchona alkaloid. UV excitation of the complex does not induce loss of specific photo fragments but to those observed in collision-induced dissociation experiments, due to the cage effect brought by the presence of bisulfate. This is due to the absence of low-energy excited charge-transfer states in the complex.
Publications
Photofragmentation mechanisms in protonated chiral cinchona alkaloids
S. Kumar, B. Lucas, J. Fayeton, D. Scuderi, I. Alata, M. Broquier, K. Barbu-Debus, V. Lepère, A. Zehnacker
Phys. Chem. Chem. Phys., 2016, vol 18, 22668-22677
Exotic Protonated Species Produced by UV-Induced Photofragmentation of a Protonated Dimer : Metastable Protonated Cinchonidine
I. Alata, D. Scuderi, V. Lepere, V. Steinmetz, F. Gobert, L. Thiao-Layel, K. Le Barbu-Debus, and A. Zehnacker-Rentien
J. Phys. Chem. A, 2015, vol 119 (39), 10007–10015
Chirality effects in gas-phase spectroscopy and photophysics of molecular and ionic complexes : contribution of low and room temperature studies
A. Zehnacker
International Reviews in Physical Chemistry, 2014, vol 33 (2), 151-207
Structural Characterization of the UV-Induced Fragmentation Products in an Ion Trap by Infrared Multiple Photon Dissociation Spectroscopy
D. Scuderi, V. Lepere, G. Piani, A. Bouchet, and A. Zehnacker-Rentien
The Journal of Physical Chemistry Letters, 2013, vol 5 (1), 56-61
Conformational Analysis of Quinine and Its Pseudo Enantiomer Quinidine : A Combined Jet-Cooled Spectroscopy and Vibrational Circular Dichroism Study
A. Sen, A. Bouchet, V. Lepere, K. Le Barbu-Debus, D. Scuderi, F. Piuzzi, and A. Zehnacker-Rentien
Journal of Physical Chemistry A, 2012, vol 116 (32), 8334-8344
Chiral Recognition in Cinchona Alkaloid Protonated Dimers : Mass Spectrometry and UV Photodissociation
D. Scuderi, P. Maitre, F. Rondino, K. Le Barbu-Debus, V. Lepere and A. Zehnacker-Rentien
J. Phys. Chem. A, 2010, vol 114 (9), 3306–3312
Effects of complexation with sulfuric acid on the photodissociation of protonated Cinchona alkaloids in the gas phase
F. Ben Nasr, I. Alata, D. Scuderi, V. Lepère, V. Brenner, N.E Jaïdane and Anne Zehnacker
PCCP, 2019,vol 21 15439-15451
Dans la même rubrique :
- Philosophie de nos études
- Dichroïsme circulaire vibrationnel (VCD)
- Dichroïsme circulaire de photoélectron (PECD)
- Spectroscopie de dipeptides cycliques
- Le rôle des liaisons faibles
- Influence de la chiralité sur la structure de polyphénylalanines
- Reconnaissance moléculaire dans les dérivés de la quinine
- Rôle de l’énergie de déformation dans la formation des complexes
- Technique et développement expérimentaux
- Activités en posters
- Collaborations





