Accueil > Équipes scientifiques > Systèmes Moléculaires, Astrophysique et Environnement (SYSTEMAE) > Offres de stages, thèses et post-docs > Photophysical investigations of molecular systems leading to Singlet Fission process
Master 2 internship
Photophysical investigations of molecular systems leading to Singlet Fission process
M2 or PhD thesis
Contact : Thomas Pino
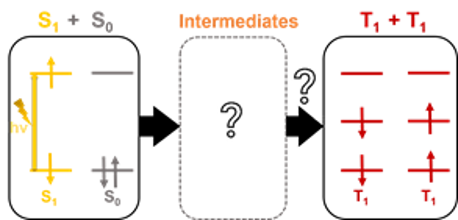
Singlet fission (SF) is a spin-allowed process involving at least two organic chromophores in which a photo-generated singlet excited state (S1S0) can spontaneously down-convert to give two lower-energy triplet states (T1+T1) with a theoretical quantum yield of triplet formation up to 200% (Fig. 1). Hence, it offers the possibility to generate two excited states by the absorption of only one photon, allowing to surpass the limit of single-junction solar cells beyond their maximum thermodynamical efficiency (Shockley–Queisser limit) of 33% to nearly 45%. SF has thus recently received a strongly growing interest for solar energy conversion. Despite numerous efforts on SF research, the number of chromophores able to generate SF is still limited and actual pathways followed by the system to reach individual triplets remain yet unclear.
Figure 1 - Illustration of the singlet fission
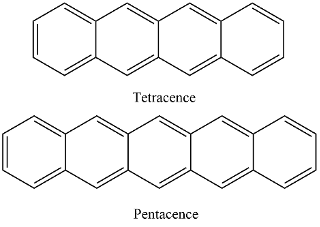
The objective of the internship is to elucidate the SF mechanism of acenes which are well known to exhibit SF (Fig. 2). Different spectroscopic techniques (steady-state absorption and emission spectroscopies as well as transient nanosecond/femtosecond transient absorption) will be employed to investigate the nature of intermediate states and mechanistic events upon photoexcitation leading to SF. Several main factors including the energetic parameter (endothermic/exothermic processes), strong/weak coupling between the singlet and triplet pair states will be considered. Understanding such fundamental processes leading to singlet fission stands as the stepping stone toward the development of more efficient photosystems.
Figure 2 - Chemical structure of several acenes
Dans la même rubrique :
- Sticking of hydrogen atom on a graphene surface
- Biocapteurs de bactéries à base de nanomatériaux fluorescents
- Energetic processing of Interstellar dust, laboratory astrophysics experimental simulations
- Unraveling the Mysteries of Cationic Species in Space : Bridging the Gap Between Astrophysical observations and Laboratory Experiments
- Wavepacket propagation applied to photoionization cross section calculation
- Photophysics of few nm-size carbonaceous particles in conditions relevant to those of the interstellar medium, toward interpretation of some astrophysical observations


