Home > Research Teams > Structure and Dynamics of Isolated Complex and Photoexcited Systems > Chirality and Spectroscopy
Chirality and Spectroscopy
Chirality is the property of molecules of not being superimposed with their mirror image. This is the case for most of the molecules related to life, like proteins, DNA, or sugars.
- The role of the topology of hydrogen-bond networks in polyfunctional systems (OH, NH2,CO). Clusters of these complex molecules involve competition between inter- and intra-molecular networks, which depend on chirality.
 The role of the conformational flexibility. The complex systems we are interested in may exist in different isomers which exhibit different complexation properties (a minor conformer may form more strongly bound complexes) and different efficiency n terms of chiral recognition.
The role of the conformational flexibility. The complex systems we are interested in may exist in different isomers which exhibit different complexation properties (a minor conformer may form more strongly bound complexes) and different efficiency n terms of chiral recognition.
 Comparison of the structure and photoreactivity of cinchona alkaloid pseudo enantiomers.
Comparison of the structure and photoreactivity of cinchona alkaloid pseudo enantiomers.
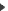 Role of the chirality of the residue in the structure of peptides.
Role of the chirality of the residue in the structure of peptides.
Contacts : The team is composed of :Anne Zehnacker-Rentien (DR CNRS), Katia Le Barbu-Debus (CR CNRS) et Valéria Lepère (MCF Univ Paris Sud).
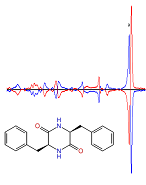
Vibrational Circular Dichroism
We conduct VCD studies on the very same systems as studied in the gas phase by laser spectroscopy.
Vibrational circular dichroism (VCD) is the weak difference in absorption between right- and left-circular polarized infrared light in chiral molecules. Besides its use for determining absolute configurations, VCD is very sensitive to conformational isomerism, molecular interactions or solvation, and thus provides a very sensitive probe of these effects. VCD studies are conducted on systems also studied in the gas phase, like cyclic dipeptides or indane derivatives.
Photon Electron circular Dichroism (PECD)
Work in progress
PECD share in common with other CD methods an exquisite sensitivity to conformational isomerism. We are currently developing, in collaboration with L. Nahon and G.Garcia (VUV DESIRS at the synchrotron SOLEIL) conformer-selective PECD methods resting on narrow-band lasers, which will simultaneously provide information on chirality and conformational landscape of chiral systems. This study is conducted in the frame of the Conf-PECD project funded by Labex PALM.
Back
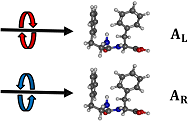
Spectrosccopy of cyclic dipeptides
Cyclic dipeptides built on a diketopiperazine (DKP) ring and containing two aromatic residues of either identical or opposite chirality are spectroscopically studied in different environments.
Role of weak molecular interactions in chiral recognition
Ionic interactions are strong interactions and dominate in ionic complexes. Also, neutral complexes are often bound by "strong "hydrogen bonds like OH…O or OH..N interactions. However, weaker interactions like OH…π or CH…π as well as delocalised dispersion interactions play an important role in chiral recognition.
We have evidenced the role of weak interaction in neutral systems on the example of (1R,2S)-(+)-cis-1-amino-2-indanol and methyl lactate.
Dispersion plays an important (…)
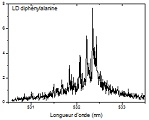
Influence of the residue’s chirality on the structure of polypetides
The structure of biopolymers like peptides or proteins depend on many factors, among them the environment, the nature of the building blocks they are made from, and their chirality. We have undertaken the study of di and tetrapetides built from phenylalanine to assess the influence of the chirality of the residue on the structure of the molecule .
Ion mobility experiments are combined with Infra-Red Multiple Photon Dissociation (IRMPD) spectroscopy and quantum chemical calculations for (…)

Chirality and photostability of quinine derivatives
Quinine derivatives belong to the cinchona alkaloid family. Cinchona alkaloids are an important class of biomolecules due to their multiple properties in the field of pharmaceutics, enantioselective catalysts, and chiral phase chromatography
They also present complex photophysical behaviours due to close-lying excited states, some of them being reactive.


