Home > Research Teams > Structure and Dynamics of Isolated Complex and Photoexcited Systems > Chirality and Spectroscopy > Role of weak molecular interactions in chiral recognition
Role of weak molecular interactions in chiral recognition
by - 17 November 2015 (modifié le 25 March 2020)
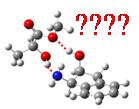
We have evidenced the role of weak interaction in neutral systems on the example of (1R,2S)-(+)-cis-1-amino-2-indanol and methyl lactate.

- Structures for the (1R, 2S) - (+) - cis-1-amino-2-indanol complex and the R (left) and S (right) methyl lactate.
Dispersion plays an important role in the Napthyl ethanol-Methyl Lactate system and Methyl Mandelate-Methyl Lactate.
Even in ionic systems such as cinchona alkaloids dimers, where the main interaction is a strong hydrogen bond (almost a shared proton), weaker interactions play an important role for discriminating pseudo enantiomers.
Publications
How do Pseudoenantiomers Structurally Differ in the Gas Phase? An IR/UV Spectroscopy Study of Jet‐Cooled Hydroquinine and Hydroquinidine
A. Sen, V. Lepere, K. Le Barbu‐Debus, and A. Zehnacker
ChemPhysChem, 2013, vol 14, 3559-3568
The role of weak hydrogen bonds in chiral recognition
D. Scuderi, K. Le Barbu-Debus, and A. Zehnacker
Phys. Chem. Chem. Phys. 2011, vol 13, 17916-179297
Chiral recognition in jet-cooled complexes of (1R,2S)-(+)-cis-1-amino-2-indanol and methyl lactate: on the importance of the CH⋯π interaction
K. Le Barbu-Debus, M. Broquier, A. Mahjoub, and A. Zehnacker-Rentien
Phys. Chem. Chem. Phys., 2009, vol 11, 7589-7598
Chiral Recognition between α-Hydroxylesters: A Double-Resonance IR/UV Study of the Complexes of Methyl Mandelate with Methyl Glycolate and Methyl Lactate
K. Le Barbu-Debus, M. Broquier, A. Mahjoub, and A. Zehnacker-Rentien
J. Phys. Chem. A, 2008, vol 112 (40), 9731–9741
Chiral recognition between lactic acid derivatives and an aromatic alcohol in a supersonic expansion: electronic and vibrational spectroscopy
N. Seurre, K. Le Barbu-Debus, F. Lahmani, A. Zehnacker, N. Borho, and M. A. Suhm
Phys. Chem. Chem. Phys., 2006, vol 8, 1007-1016


